References Kiwa
What others say about our products
Kiwa Assurance B.V. has been designated by the Ministry of Health, Welfare and Sport as Notified Body, since the end of 2015. Medical Certifications tries to make sure that the medical devices are safe before they are being used in the health care sector. Before this is achieved a series of assessments, tests and measurements need to be completed.
The Medical Device Regulation applies to all medical devices and accessories which come with the medical device. Devices are split up in different risk classes which are assessed and tested according to the relevant requirements.
References
Kiwa performs conformity assessments for compliance with the Medical Device Regulation and EN-ISO 13485 for manufacturers of active medical devices and software. These references can be found below.
FEops nv - insight for excellence
Innovative software to support non-invasive cardiac interventions
"Over the past period, Kiwa Dare has checked and approved our quality system (ISO 13485, MDR) and our technical file.
During this process, there was constructive interaction between the Notified Body (Kiwa Dare) and the company (FEops), which fully benefits patient safety".
"Since our previous Notified Body did not receive accreditation for MDR, we have taken the step to Kiwa Dare to enter the European market as a class IIa device under the MDR".
“For us, the MDR was the trigger to switch to Kiwa Dare, as our NB was not accredited under MDR. The route to MDR was not easy, but we got through it fairly quickly and have never regretted the switch from NB”
"We are very satisfied with the cooperation with Kiwa Dare!! Kiwa Dare's reviews and audits are very professional, and allow us to take our processes to a higher level. In addition, there is very smooth communication, which is of course crucial to avoid project delays."
Franky Dubois / QA/RA Manager FEops
FEops nv 
FEops was founded in 2009, with an initial focus on providing advice to developers of heart implants. From 2018 the software FEops HEARTguide was placed on the European market as a class I device under the MDD. This software supports cardiologists in performing non-invasive cardiac interventions.
Later we also added the United States via the De Novo route.
FEops HEARTguideTM
FEops HEARTguideTM supports physicians, providing better insights through patient-specific simulations for two procedures.
TAVI - Transcatheter Aortic Valve Implantation 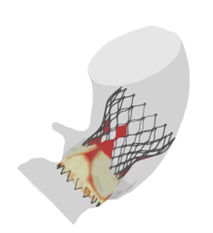
- This is a replacement of the aortic valve using a catheter inserted through the femoral artery.
- FEops provides additional insights here with regard to the dimensions and position of these implants in the anatomy of the patient.
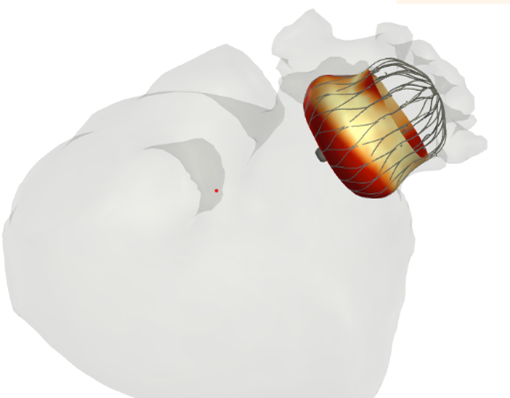
LAAO – Left Atrial Appendage Occlusion
- Is a treatment to reduce the risk of clotting in the atrial appendage of the left atrium and thereby limit the risk of stroke related to atrial fibrillatio
- FEops provides better insights into the interaction of the implant (size and position) with the anatomical structure of the patient.
More information about FEops
T +32 9 292 80 30
E info@feops.com